What is Community
A community is an assemblage of two or more populations of organisms occupying the same geographical area.
Example of a pond community
Ecosystem: a system formed by the interaction of a community of organisms with their environment.
Habitat: the natural environment of an organism; place that is natural for the life and growth of an organism
Community: a group of interdependent plants and animals inhabiting the same region and interacting with each other through food and other relationships
Population: All individuals belong to the same species.
Habitat: The environment in which the populations of organisms thrive in is called the habitat
Ecosystem: Together, the habitat and the communities residing in it forms an Ecosystem.
Factors Influencing Ecology
Abiotic:
-Temperature
-Rainfall
-Light Intensity
-Salinity
-Humidity
-Wind speed
-Wave action
-pH
Biotic factors
Abundance
Presence of predators
Competition
Ecological Interactions
Mutualism
Mutualism: A symbiotic relationship between individuals of different species in which both individuals benefit from the association.
Example of Mutualism
Clownfish and Sea anemone
Ants and Aphids
Cleaner Shrimps and Eel
Egyptian plover and Crocodile
Commensalism
Commensalism: A form of symbiosis between two organisms of different species in which one of them benefits from the association whereas the other is largely unaffected or not significantly harmed or benefiting from the relationship.
Examples of Commensalism
Epiphytes and Rain tree
Cattle Egret and Cattle
Remora and Shark
Barnacles and Scallop
Predation
A form of symbiotic relationship between two organisms of unlike species in which one of them acts as predator that captures and feeds on the other organism that serves as the prey
Example: Cheetah and Thompson Gazelle
Herbivory
The consumption of herbaceous vegetation. The consumer benefits while the producer suffers.
Example: Zebra and Grass
Parasitism
A form of symbiosis in which one organism (called parasite) benefits at the expense of another organism usually of different species (called host). The association may also lead to the injury of the host.
Example: Tree-ear Fungus and Tree
Predation and Parasitism
A combination of both
Example: Mosquito, Malaria parasite and Man
Science E-Portfolio
Search This Blog
Tuesday, August 30, 2011
Thursday, July 14, 2011
Endoscopes
An endoscope can consist of
• a rigid or flexible tube
• a light delivery system to illuminate the organ or object under inspection. The light source is normally outside the body and the light is typically directed via an optical fiber system
• a lens system transmitting the image to the viewer from the objective lens to the viewer, typically a relay lens system in the case of rigid endoscopes or a bundle of fiber optics in the case of a fiberscope
• an eyepiece
• an additional channel to allow entry of medical instruments or manipulators
Before the fiber optics endoscope was invented, they used a semi-flexible tube with a tiny camera attached at the tip to detect stomach ulcers and stomach cancer in its early stage. The first fiber optics endoscope was invented in 1963/64. It paved the way for key-hole surgery. A “fibroscope” is actually a bundle of glass fibres that is able to channel light from one end to the other through total internal reflection.
However, the fiber optics endoscope was not the latest version of endoscope invented. The Rod-lens Endoscopes was one of the more advanced versions. This is because there were many difficulties while using the fiber optics endoscope and 50000 fibres give only a 50000pixel image, therefore, if any fiber breaks, that means that the image would decrease in pixels and quality. After continued flexingm more and more fibres would break and eventually, the whole bundle has to be replaced and it is very expensive. Therefore, the elegant solution that Hopkins produced (in the late 1960s) was to fill the air-spaces between the 'little lenses' with rods of glass. These fitted exactly the endoscope's tube - making them self-aligning and requiring of no other support and allowed the little lenses to be dispensed with altogether. The rod-lenses were much easier to handle and utilized the maximum possible diameter available. With the appropriate curvature and coatings to the rod ends and optimal choices of glass-types, all calculated and specified by Hopkins, the image quality was transformed - even with tubes of only 1mm. in diameter. With a high quality 'telescope' of such small diameter, the tools and illumination system could be comfortably housed within an outer tube. Once again, it was Karl Storz who produced the first of these new endoscopes as part of a long and productive partnership between the two men. Whilst there are regions of the body that will forever require flexible endoscopes (principally the gastrointestinal tract), the rigid rod-lens endoscopes have such exceptional performance that they are to this day the instrument of choice and in reality have been the enabling factor in modern key-hole surgery. (Harold Hopkins was recognized and honoured for his advancement of medical-optic by the medical community worldwide. It formed a major part of the citation when he was awarded the Rumford Medal by the Royal Society in 1984.)
Adapted from Wikipedia.
• a rigid or flexible tube
• a light delivery system to illuminate the organ or object under inspection. The light source is normally outside the body and the light is typically directed via an optical fiber system
• a lens system transmitting the image to the viewer from the objective lens to the viewer, typically a relay lens system in the case of rigid endoscopes or a bundle of fiber optics in the case of a fiberscope
• an eyepiece
• an additional channel to allow entry of medical instruments or manipulators
Before the fiber optics endoscope was invented, they used a semi-flexible tube with a tiny camera attached at the tip to detect stomach ulcers and stomach cancer in its early stage. The first fiber optics endoscope was invented in 1963/64. It paved the way for key-hole surgery. A “fibroscope” is actually a bundle of glass fibres that is able to channel light from one end to the other through total internal reflection.
However, the fiber optics endoscope was not the latest version of endoscope invented. The Rod-lens Endoscopes was one of the more advanced versions. This is because there were many difficulties while using the fiber optics endoscope and 50000 fibres give only a 50000pixel image, therefore, if any fiber breaks, that means that the image would decrease in pixels and quality. After continued flexingm more and more fibres would break and eventually, the whole bundle has to be replaced and it is very expensive. Therefore, the elegant solution that Hopkins produced (in the late 1960s) was to fill the air-spaces between the 'little lenses' with rods of glass. These fitted exactly the endoscope's tube - making them self-aligning and requiring of no other support and allowed the little lenses to be dispensed with altogether. The rod-lenses were much easier to handle and utilized the maximum possible diameter available. With the appropriate curvature and coatings to the rod ends and optimal choices of glass-types, all calculated and specified by Hopkins, the image quality was transformed - even with tubes of only 1mm. in diameter. With a high quality 'telescope' of such small diameter, the tools and illumination system could be comfortably housed within an outer tube. Once again, it was Karl Storz who produced the first of these new endoscopes as part of a long and productive partnership between the two men. Whilst there are regions of the body that will forever require flexible endoscopes (principally the gastrointestinal tract), the rigid rod-lens endoscopes have such exceptional performance that they are to this day the instrument of choice and in reality have been the enabling factor in modern key-hole surgery. (Harold Hopkins was recognized and honoured for his advancement of medical-optic by the medical community worldwide. It formed a major part of the citation when he was awarded the Rumford Medal by the Royal Society in 1984.)
Adapted from Wikipedia.
How are mirages formed?
Mirages are a result of refraction. They are often sighted in deserts. Some people might call it hallucination but there is a reason why there can be an “oasis” appearing in the middle of a desert when there actually in not one. I have been wondering how they are formed and I did not really understand the explanation. However, now that we have learnt on refraction, I now can relate a little better.
Mirages are formed when light rays are bent as the light ray passes through air layers with different densities. This is actually refraction. The reason why mirages can be form due to varying densities of air is because the index of refraction for air varies with the density of the air. Air density is dependent on its pressure, temperature and water vapour content. Air density is also proportional to its pressure, which means that density increases as pressure increases and it is also inversely proportional to its temperature, which means that density decreases as temperature increases.
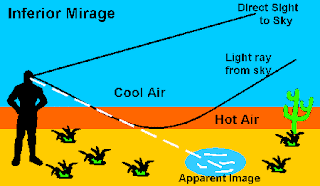
This image is trying to express that the “oasis” that the eye apparently sees is actually a mirage. The mirage of the oasis is actually the image of the bluish sky being strongly refracted by the hot air near the surface so that it appears to be water lying on the surface. This is also known as an inferior mirage as the warm air is below the cool air.
The opposite of an inferior mirage is a superior mirage. It occurs when the cool air is under the warm air. It happens very often at sea , or over ice and snow surfaces. When cold air lies below the warm air, the light rays from the object is bent towards our eyes, tricking it into thinking that the object is higher or taller in appearance than it actually is. It can also make objects appear as though it is floating. Here are some examples:
Sources, Help and Images:
http://www.islandnet.com/~see/weather/elements/mirage1.htm
Mirages are formed when light rays are bent as the light ray passes through air layers with different densities. This is actually refraction. The reason why mirages can be form due to varying densities of air is because the index of refraction for air varies with the density of the air. Air density is dependent on its pressure, temperature and water vapour content. Air density is also proportional to its pressure, which means that density increases as pressure increases and it is also inversely proportional to its temperature, which means that density decreases as temperature increases.
This image is trying to express that the “oasis” that the eye apparently sees is actually a mirage. The mirage of the oasis is actually the image of the bluish sky being strongly refracted by the hot air near the surface so that it appears to be water lying on the surface. This is also known as an inferior mirage as the warm air is below the cool air.
The opposite of an inferior mirage is a superior mirage. It occurs when the cool air is under the warm air. It happens very often at sea , or over ice and snow surfaces. When cold air lies below the warm air, the light rays from the object is bent towards our eyes, tricking it into thinking that the object is higher or taller in appearance than it actually is. It can also make objects appear as though it is floating. Here are some examples:
Sources, Help and Images:
http://www.islandnet.com/~see/weather/elements/mirage1.htm
Refraction and Total Internal Reflection
This is one of the subtopics of Light. I felt this part a little confusing as it was challenging to draw the total internal reflection and the refraction digrams.
Refraction of Light
When light strikes an opaque reflecting surface such as a irror, most of the light is reflected. Some light, however, is absorbed by the surface.
At the air-glass surface (or interface), light is partially reflected off the surface and partially transmitted through the medium. THe reflected light follows the laws of reflection studied earlier. The light that is transmitted through the medium as it travels from one optical medium (air) into another (glass). This bending effect of light is known as refraction.
The amount of refraction depends on the optical density of the medium. Here we define optical density to be a measure of the exten to which a substance transmit visible light. The higher the opticaldensity of a medium, the lower the tranmittance, thus the greater is the refraction of light in it. For example, glass is an optically denser medium than water. If a light ray were to enter glass from air and water from air respectively, keeping all other parameters constant, the ray would bend more in glass than in water.
However, that the bending only occurs at the interface. Whithin the same medium, light still travels in a straight line.
How will light behave when it enters a different optical medium?
When a light ray strikes perpendically to the surface of an opticalmedium e.g. glass, it passes straight through without refraction.
This is because when it travels from air into glass, its angle of incidence is zero, thus, its angle of refraction is also zero. Even though light is not bent, its speed stilldecreases when it enters the optically denser medium, glass.
The angle of refraction is always smaller than the angle of incidence. This means that when a light ray travels from an optically less dense medium into an optically denser medium at an angle, it is always refracted towards the normal as light travels slower in the optically denser medium.
Refraction of Light
When light strikes an opaque reflecting surface such as a irror, most of the light is reflected. Some light, however, is absorbed by the surface.
At the air-glass surface (or interface), light is partially reflected off the surface and partially transmitted through the medium. THe reflected light follows the laws of reflection studied earlier. The light that is transmitted through the medium as it travels from one optical medium (air) into another (glass). This bending effect of light is known as refraction.
The amount of refraction depends on the optical density of the medium. Here we define optical density to be a measure of the exten to which a substance transmit visible light. The higher the opticaldensity of a medium, the lower the tranmittance, thus the greater is the refraction of light in it. For example, glass is an optically denser medium than water. If a light ray were to enter glass from air and water from air respectively, keeping all other parameters constant, the ray would bend more in glass than in water.
However, that the bending only occurs at the interface. Whithin the same medium, light still travels in a straight line.
How will light behave when it enters a different optical medium?
When a light ray strikes perpendically to the surface of an opticalmedium e.g. glass, it passes straight through without refraction.
This is because when it travels from air into glass, its angle of incidence is zero, thus, its angle of refraction is also zero. Even though light is not bent, its speed stilldecreases when it enters the optically denser medium, glass.
The angle of refraction is always smaller than the angle of incidence. This means that when a light ray travels from an optically less dense medium into an optically denser medium at an angle, it is always refracted towards the normal as light travels slower in the optically denser medium.
Monday, May 23, 2011
Acids and Bases
We began on a new topic Acids and Bases. For me, it was a very challenging topic as I had difficulty memorising the chemical names and formulas. It took a lot of revision to grasp the concept.
To begin, an acid is a substance which produces hydrogen ions as the only positive ions when it is dissolved in water. Some examples of strong acids are Hydrochloric acid, Nitric acid and Sulfuric acid. On the other hand, some examples of weak acids are ethanoic acid and citric acid. The strength of an acid depends on its degree of dissociation / ionisation in water to form hydrogen ions.
Strong Acids
Strong acids are acids that ionise / dissociate completely in water to produce hydrogen ions (H+).
There are no molecules left.
The properties and reactions of acids are due to these hydrogen ions.
Weak Acids
A weak acid is one that ionises / dissociates incompletely / partially in water to produce few hydrogen ions (H+).
Most of the acid molecules remain as molecules.
Concentration
A concentrated acid is one in which the proportion of acid to water is very high.
A diluted acid is one in which the proportion of acid to water is low.
The concentration of an acid does not affect the strangth of an acid.
Importance of water for acidity
Pure acids exist as molecules instead of ions.
Pure acids do not behave as acids as the properties of acids are due to the presence of hydrogen ions.
When acids are mixed with water, ionisation of acids occurs, and hydrogen ions are produced. Therefore, acids can only behave as acids when they are dissolved in water.
Properties of Acids
Acids have a sour taste.
Acids dissolve in water to form colourless solutions which conduct electricity.
Acids turn blue litmus red.
Reactions
Acids react with metals, carbonates & bases
Acids react with metals to produce salt and hydrogen
(However, metals less reactive than hydrogen is not reactive enough to react with acids.)
Acids react with carbonates to produce salt, carbon dioxide and water
Acids react with bases to produce salt and water
pH
pH values depend on the concentration of acid/base and degree of dissociation. The use of pH in measuring the strength of an acid is limited since its value changes with concentration.
To begin, an acid is a substance which produces hydrogen ions as the only positive ions when it is dissolved in water. Some examples of strong acids are Hydrochloric acid, Nitric acid and Sulfuric acid. On the other hand, some examples of weak acids are ethanoic acid and citric acid. The strength of an acid depends on its degree of dissociation / ionisation in water to form hydrogen ions.
Strong Acids
Strong acids are acids that ionise / dissociate completely in water to produce hydrogen ions (H+).
There are no molecules left.
The properties and reactions of acids are due to these hydrogen ions.
Weak Acids
A weak acid is one that ionises / dissociates incompletely / partially in water to produce few hydrogen ions (H+).
Most of the acid molecules remain as molecules.
Concentration
A concentrated acid is one in which the proportion of acid to water is very high.
A diluted acid is one in which the proportion of acid to water is low.
The concentration of an acid does not affect the strangth of an acid.
Importance of water for acidity
Pure acids exist as molecules instead of ions.
Pure acids do not behave as acids as the properties of acids are due to the presence of hydrogen ions.
When acids are mixed with water, ionisation of acids occurs, and hydrogen ions are produced. Therefore, acids can only behave as acids when they are dissolved in water.
Properties of Acids
Acids have a sour taste.
Acids dissolve in water to form colourless solutions which conduct electricity.
Acids turn blue litmus red.
Reactions
Acids react with metals, carbonates & bases
Acids react with metals to produce salt and hydrogen
(However, metals less reactive than hydrogen is not reactive enough to react with acids.)
Acids react with carbonates to produce salt, carbon dioxide and water
Acids react with bases to produce salt and water
pH
pH values depend on the concentration of acid/base and degree of dissociation. The use of pH in measuring the strength of an acid is limited since its value changes with concentration.
Term 1 Test
I scored 31.5 for my Term 1 Test on the periodic table, atomic structure and word equations. Although it was an A1, I felt that there was much room for improvement and I could have made less careless mistakes. One of the areas in which I need improvement is being specific. I loss one mark as I was not specific enough. Secondly, I tend to misintepret the question and at times, the answers I gave were incorrect even though I know how to do the question. I would definitelt try harder the next test.
Tuesday, March 8, 2011
Atomic Structure
After Periodic Table, we began on atomic structure. To help us in this topic, besides the usual notes, worksheet and assignment, we were also provided a powerpoint to help us digest all this infomation at home if we did not understand what the teacher was teaching in class. Here are some of the notes.
The number of protons in an atom is called the proton number. Proton number is also known as the Atomic Number.
Nucleon number is the number of protons and neutrons in the nucleus of an atom. Nucleon number is also called the Mass Number.
Nucleon (Mass) number = number of protons + number of neutrons
The centre of an atom is called the nucleus which contains the protons and neutrons.
The electrons in an atom are arranged in shells (orbits) at different distances from the nucleus.
Note: Shells are also called energy levels.
Each shell can hold a certain maximum number of electrons.
(a) 1st shell - 2 electrons
(b) 2nd shell - 8 electrons
(c) 3rd shell - 8 electrons
(1st 20 elements only)
Advanced: For elements after calcium in the 4th period, their third shell can hold up to 18 electrons.
An atom can be described as an electrically neutral entity made up of a positively charged nucleus at its centre with negatively charged electrons moving around the nucleus.
All atoms of the same element have the same number of protons while those of different elements contain different number of protons.
Isotopes are atoms of the same element with different numbers of neutrons.
Only valence electrons are involved in chemical reactions.
Following this topic on atomic structure, it would go further and touch on the formation on ions, as well as chemical bonding.
The number of protons in an atom is called the proton number. Proton number is also known as the Atomic Number.
Nucleon number is the number of protons and neutrons in the nucleus of an atom. Nucleon number is also called the Mass Number.
Nucleon (Mass) number = number of protons + number of neutrons
The centre of an atom is called the nucleus which contains the protons and neutrons.
The electrons in an atom are arranged in shells (orbits) at different distances from the nucleus.
Note: Shells are also called energy levels.
Each shell can hold a certain maximum number of electrons.
(a) 1st shell - 2 electrons
(b) 2nd shell - 8 electrons
(c) 3rd shell - 8 electrons
(1st 20 elements only)
Advanced: For elements after calcium in the 4th period, their third shell can hold up to 18 electrons.
An atom can be described as an electrically neutral entity made up of a positively charged nucleus at its centre with negatively charged electrons moving around the nucleus.
All atoms of the same element have the same number of protons while those of different elements contain different number of protons.
Isotopes are atoms of the same element with different numbers of neutrons.
Only valence electrons are involved in chemical reactions.
Following this topic on atomic structure, it would go further and touch on the formation on ions, as well as chemical bonding.
Subscribe to:
Comments (Atom)